一、行业背景:潜力与痛点并存的水系Zn//MnO₂电池 在大规模储能领域,可充电水系Zn//MnO₂电池凭借两大核心优势成为研究热点: - 高安全性:采用水系电解液,彻底规避有机电解液的燃爆风险,适配家庭储能、电网储能等对安全要求高的场景; - 高成本效益:Zn(锌)和MnO₂(二氧化锰)原料储量丰富、价格低廉,无需依赖稀有金属,具备规模化量产基础。 但它的商业化进程被一个关键问题卡住——Zn负极稳定性不足。在酸性电解液中,Zn负极会同时发生“酸性腐蚀”和“析氢反应(HER)”:前者消耗电极材料,后者产生的氢气会堵塞电极孔隙,双重作用下电池寿命大幅缩短,成为制约其落地的核心瓶颈。 二、核心方案:微量M4的“双重保护”破解负极难题 针对上述痛点,清华大学深圳国际研究生院周光敏团队提出了一种“简单却高效”的解决方案——在电解液中引入微量对羟基苯甲醛(代号M4) ,仅通过分子级调控就实现了Zn负极的稳定保护。 对羟基苯甲醛的作用并非单一机制,而是通过“双重协同”解决问题,其核心逻辑源于对Zn²⁺和H₂O的强亲和力: 1. 第一层保护:重构Zn²⁺溶剂化鞘 对羟基苯甲醛分子会取代[Zn(H₂O)₆]²⁺(锌离子水合结构)中的一个水分子,形成新的溶剂化结构。这一重构减少了自由水分子与Zn负极的接触,从源头降低了酸性腐蚀和析氢反应的发生概率; 2. 第二层保护:吸附形成界面屏障 对羟基苯甲醛分子会主动吸附在Zn负极表面,形成一层致密的“保护膜”。这层膜能直接阻隔H⁺(氢离子)与Zn的直接反应,同时改变电解液的氢键网络、降低水分子活性,进一步强化界面稳定性。 三、机制解析:M4如何从分子层面优化电池环境? 团队通过理论计算与实验验证,明确了M4提升电池性能的分子机制,核心可总结为两点: - 结合能调控:数据显示,Zn²⁺与M4的结合能、H₂O与M4的结合能,均强于传统溶剂化结构中的相互作用,这让M4能稳定“占据”溶剂化鞘和电极界面,挤压有害反应的发生空间; - 氢键网络优化:M4的加入改变了电解液中水分子的氢键网络,减少“自由水分子”数量——这类水分子正是酸性腐蚀和析氢反应的“帮凶”,其活性降低后,电解液整体稳定性显著提升。 四、实验数据:四大图组验证性能跨越式提升 团队通过四组核心实验(对应图1-图4),从“电解液特性→负极保护→电化学稳定性→电池性能”全链条验证了M4的效果,关键数据如下:
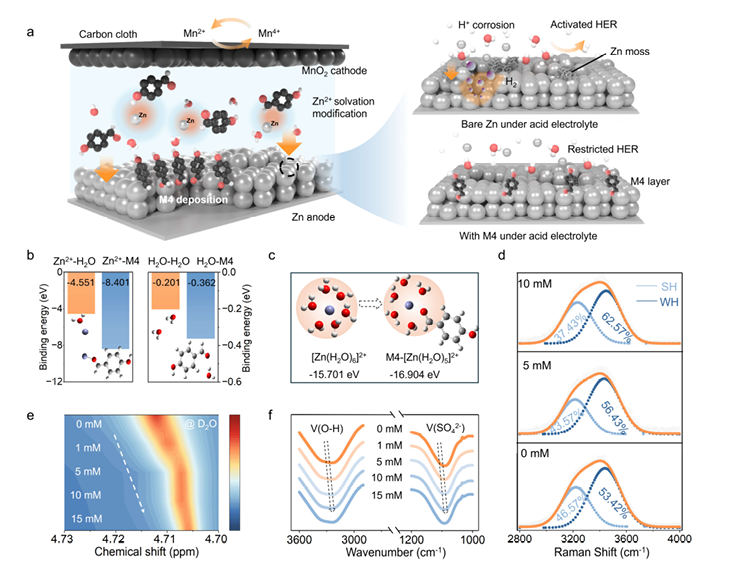
图1:电解液特性表征——证明M4的分子级调控作用 该图组聚焦“M4如何改变电解液环境”,核心结论包括: - (a) 清晰展示电池储能机制、副反应(腐蚀/HER)及M4优化策略,直观呈现解决方案; - (c) 对比添加M4前后的Zn²⁺溶剂化结构,证实M4成功取代水分子、重构溶剂化鞘; - (d)(e)(f) 通过红外、核磁等表征,验证M4改变了电解液氢键网络,降低了自由水分子活性。

图2:Zn负极保护评估——阻断腐蚀与析氢 该图组直接测试M4对Zn负极的保护效果,关键结果有: - (a) 浸泡实验显示,添加M4的Zn箔质量保持率更高、电解液pH值更稳定,证明腐蚀被抑制; - (g) 原位光学显微镜观察到,M4组氢气气泡显著减少,析氢反应被有效阻断; - (d)(f) Tafel曲线、LSV曲线证实,M4降低了腐蚀反应和HER的动力学速率。
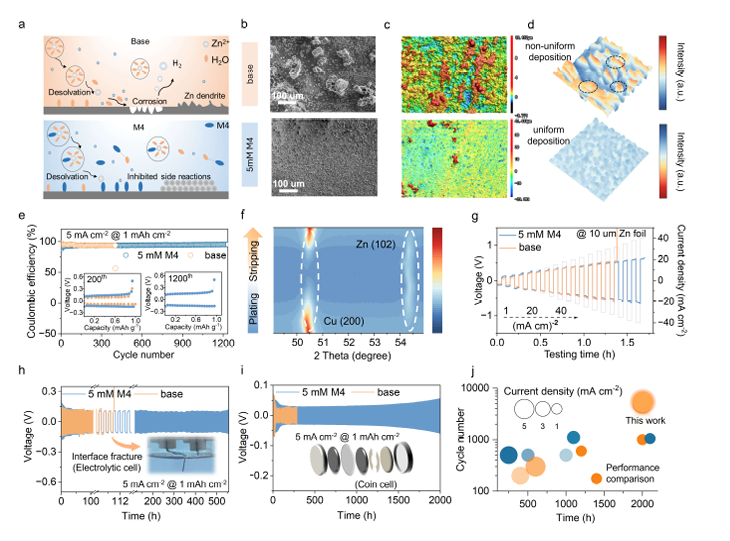
图3:Zn负极电化学稳定性——实现长寿命循环 该图组聚焦Zn负极的循环可逆性,核心数据包括: - (h)(i) Zn对称电池在M4电解液中,循环稳定性超2000小时(5 mA cm⁻²、1 mAh cm⁻²),寿命是传统电解液的5倍; - (e) Zn//Cu电池库仑效率稳定,证明Zn沉积/剥离过程可逆性大幅提升; - (b)(c)(d) 循环后Zn负极的SEM、CLSM图像显示,M4组电极表面更平整,无明显腐蚀痕迹。
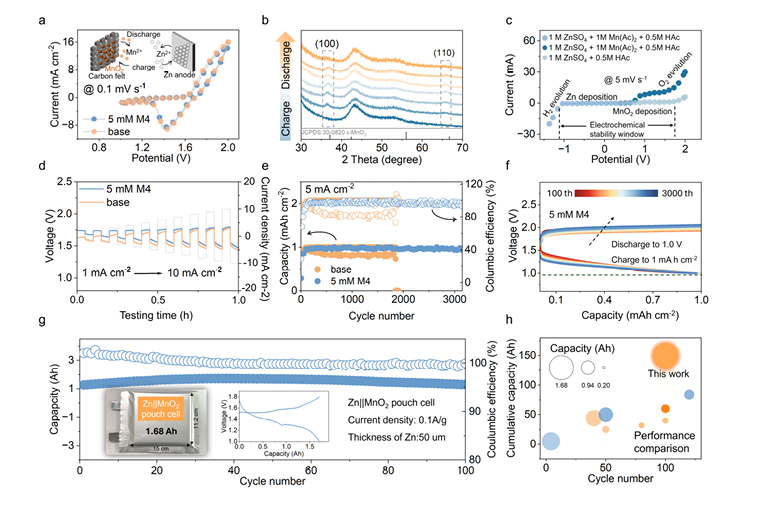
图4:Zn//MnO₂电池性能——从扣式到软包的突破 该图组验证了M4对完整电池的性能提升,亮点如下: - (e) Zn//MnO₂扣式电池在M4电解液中,稳定循环超3000次,平均库仑效率达97.3%; - (g) 容量1.68 Ah的Zn//MnO₂软包电池(尺寸12.2×15 cm²),在0.1 Ag⁻¹下稳定运行超100次,验证实际应用潜力; - (h) 性能对比显示,该成果远超此前报道的水系Zn//MnO₂电池水平。 五、研究意义与结论:为水系储能开辟新路径 本研究的核心价值,在于提供了一种“简单、低成本、可规模化”的水系电池优化策略——无需复杂的电极结构设计,仅通过微量分子添加剂(M4),就能从分子层面解决Zn负极的根本问题。 其关键结论可总结为三点: 1. 对羟基苯甲醛(M4)通过“重构溶剂化鞘+界面吸附”双重机制,同时抑制了Zn负极的酸性腐蚀和析氢反应; 2. 该策略实现了电池性能的跨越式提升:对称电池循环超2000小时,Zn//MnO₂电池循环超3000次; 3. 软包电池的稳定表现,证明该方案具备实际应用潜力,为大规模水系储能系统的开发提供了新方向。 永津集团愿景:对羟基苯甲醛助力下一代储能发展 永津集团认为,对羟基苯甲醛在 Zn//MnO₂电池中的成功应用,不仅是储能领域的一项突破,更是精细化工材料与新能源跨领域融合的典范。未来,永津集团将从两个关键方向推进对羟基苯甲醛的创新应用: 其一,优化对羟基苯甲醛生产工艺以降低成本,同时开发高纯度、低杂质的 “储能级” 产品,满足新能源技术的需求;其二,加强与科研机构及电池企业的合作,探索对羟基苯甲醛在其他水系电池(如 Zn//V₂O₅电池、Zn// 普鲁士蓝类似物电池)中的应用,助力更多储能技术突破性能瓶颈。 作为对羟基苯甲醛领域的专业生产企业,永津集团将以技术创新和高品质产品为支撑,推动行业高质量发展,为储能、医药、化工等领域的进步注入动力。 论文信息来源:Liu, Y., Liu, Z., Xiao, Z., Lao, Z., Liu, J., Xiao, X., Fu, Q., Zheng, F., & Zhou, G. (2025). Suppressing spontaneous acidic corrosion and hydrogen evolution for stable Zn//MnO₂ batteries. Angewandte Chemie International Edition.
